Forums › Laser Resources › Laser Physics Related Literature › Near-Infrared Dental Diode Lasers: Scientific and Photobiolo
- This topic is empty.
-
AuthorPosts
-
AnonymousGuestNear-Infrared Dental Diode Lasers: Scientific and Photobiologic Principles and Applications
Eric Bornstein DMD
ABSTRACT
In the past few years, the use of diode lasers in clinical dentistry has increased considerably, but there needs to be more research concerning the events that occur when the tip of the laser comes into contact with oral tissues. When an unclad “naked’ fiber tip comes in contact with tissue and fluid, debris will immediately accumulate on the tip, and this debris will absorb the intense infrared laser energy propagating through the fiber, which will cause the tip to heat and immediately carbonize. As the energy from the infrared laser photons continue to be absorbed by this newly carbonized tip, the tip will become red hot (above 726◦ C). This secondary emission of the “hot tip” energy to the tissue is accompanied by fundamentally different heat transfer and photobiologic events to the oral tissues and fluids (including blood). The purpose of this paper is to describe the quantum and thermodynamic events associated with energy emission from diode lasers with “hot contact tips”, and the tissue response to this contact with the laser. Only with an understanding of these concepts can clinicians make the most appropriate choices for diode laser treatment of their patients.INTRODUCTION
When a clinician decides to use a laser as part of treatment, it is essential that the user has a clear understanding of the discipline and applications of photobiology. Laser photobiology is the science describing the unique interactions of nonionizing electromagnetic radiation (laser generated photons) with tissues and the molecular components that comprise tissues (i.e., hemoglobin, water, collagen). As an example, the Er:YAG laser is one of the more commonly used lasers in dentisty today, and produces a beam at 2.94um in the mid infrared portion of the electromagnetic spectrum. At this wavelength, the Er:YAG has the highest coefficient of absorption for water in the mid-infrared range and correspondingly, the lowest depth of tissue penetration. (1) To effectively cut or ablate hard or soft tissue, the Er:YAG laser targets the chromophore of water selectively instead of the extracellular matrix of collagen or hydroxapetite, and produces an instantaneous vaporization of the water to a depth of about 4 microns/pulse (four 1000ths of a millimeter) in front of the beam. (2) The result of this interaction is a thermally driven instantaneous controlled tissue degradation or ablation, with an explosive ejection of the degraded cellular components and heated vaporous material, with poor heamostasis in soft tissue. (2)
A second example is the superpulsed CO2 laser is a gas laser that produces a beam at 10.6um in the far infrared portion of the electromagnetic spectrum. At this wavelength, the super-pulsed CO2 has the highest coefficient of absorption for water in the far-infrared range and correspondingly has a penetration depth of about 50um with a greater thermal interaction and diffusion than the Er:YAG. (1) The superpulsed CO2 is an excellent laser for hemostasis and coagulation as opposed to the Er:YAG which is used for cutting and thermal mechanical ablation without significant tissue thermal diffusion to surrounding tissues. (3)
The focus of this paper, Diode lasers, have a wide variety of medical and dental applications. Current diode technology can emit photons over a wide range of the electromagnetic spectrum, ranging from the red visible (600-700nm) to the near infrared (800-1000nm). The wavelength generated by the diode laser is related to the diode formed from different concentrations and layers of materials, such as gallium-arsenide and gallium-aluminum-arsenide.(1) Currently available FDA approved diode lasers for dental applications have a photobiology that is always based on thermal tissue interactions, but is fundamentally different from other dental hard and soft tissue lasers, by the degree of thermal interaction based on energy penetration in the tissues. (4) If the wavelength of the Er:YAG (2.94µm) is compared to conventional dental diode soft tissue lasers (810nm to 980nm wavelength), the depth of penetration per pulse with diodes is estimated to be greater by a factor of 104 (10,000x) or 4cm. (4) A diode laser is a solid-state laser with completely different chromophore targeting and absorption characteristics than the Er;YAG . Diode lasers have shorter wavelengths (800+ nm vs 2.94µm ), and thus have high absorption peaks in melanin and hemoglobin. This will cause the laser energy to essentially pass through the interstitial and intracellular water, and produce thermal haemostatic and necrotic effects much deeper in the tissues as the photons are absorbed by the tissue pigments. Hence, the Er:YAG laser is safer and more controlled for purposes of cutting tissue like bone, but does not have good haemostatic properties like the Diode. (5)
Before using a diode laser a clinician should understand the energy being delivered, the target of the laser energy (darker pigments), and how much laser energy is needed to achieve a specific task, in a controlled fashion. In addition, the clinician needs to understand how the energy changes once the laser delivery fiber comes in contact with oral tissues and/or fluids, including blood. This knowledge is necessary to achieve the desired effect, while minimizing collateral damage to otherwise healthy tissue. Figure 1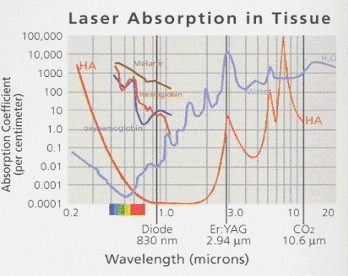
Figure 1 Laser energy Absorption graphPhotobiology of Near-infrared Laser Energy
Niemz (4) has determined that all effects with near-infrared laser wavelengths at pulse durations of 1 microsecond or greater are thermal in nature. There are 5 factors to consider regarding heat generation by these lasers:
1) Wavelength and optical penetration depth of the laser
2) Absorption charactistics of exposed tissue
3) Temporal mode (pulsed or continuous)
4) Exposure time
5) Power density of the laser beamThe first parameter of near-infrared diode lasers that must be understood is the penetration depth of the optical energy. Diode lasers in the near infrared range have a very low absorption coefficient in water, hence they achieve deep optical penetration in tissues that contain 80% water (including the oral mucosa and gingiva). This means that for a conventional dental diode soft tissue laser ( as stated earlier), the depth of penetration per pulse is estimated to be greater than the Er:YAG hard tissue laser by a factor of 104. (4) The shorter wavelengths of the near-infrared diode and Nd:YAG lasers have very high absorption peaks in molecules (chromophores) such as melanin and hemoglobin. This will allow the laser energy to pass with minimal absorption through water, producing thermal effects much deeper in the tissue (up to 4cm) as the photons are absorbed by the deeper tissue pigments. This photobiology allows for controlled deeper soft-tissue coagulation, as the photons that emerge (in a cone pattern of energy) from the distal end of a near-infrared diode laser fiber are absorbed by blood and other tissue pigments (Figures. 2, 3). It should be noted that there are respective absorption, tissue temperature, and thermal effect gradients within this illuminated volume or cone of tissue.
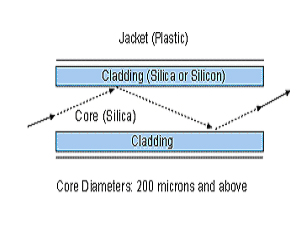
Figure 2 Optical Fiber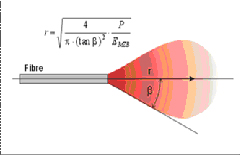
Figure 3 “Cone” Dispersion Pattern
The next parameter to bear in mind is the Heat Effects on the tissue being irradiated,
based on the Pulse Mode of currently available near-infrared systems. Presently, for
dental treatment, near-infrared lasers either emit photons in the Continuous Wave (CW)
or Gated CW Pulsed Mode for Diode systems, and Free Running Pulsed (FRP) for
Nd:YAG’s . This fact is very important to comprehend, because the length
(duration) of the tissue exposure to the photon energy of the laser will govern the thermal
tissue interaction that is achieved.
In the CW or Gated CW mode, laser photons are emitted at one single power level, in a continuous stream. When the stream is Gated, there is an intermittent shuttering of the beam, as a mechanical gate is positioned in the path of the beam, essentially turning the laser energy on and off. The duration of on and off times, of this type of laser system is generally on the order of milliseconds (1 millisecond = 1/1000th of a second), and the “power-per-pulse” stays at the average power of the CW beam. Nd:YAG lasers (in the FRP mode) can produce very large peak energies of laser energy, for extremely short time intervals on the order of microseconds (1 microsecond = 1/1,000,000th sec). As an example, one of these lasers with a temporal pulse duration of 100 microseconds, with pulses delivered at ten per second (10 Hz), would mean that the laser photons are hitting the tissue for only 1/1000th of a second (total time) and the laser is “off” for the remainder of that second. This will give the tissue significant time to cool before the next pulse of laser energy is emitted. These longer intervals between pulses will benefit the thermal relaxation time of the tissue. The CW mode of operation will always generate more heat than a pulsed energy application. (4)If the temporal pulses are to long (or the exposure in CW is to long), the thermal
relaxation effect in the tissues is overcome and irreversible damage to non-target areas
can occur. If adequate cooling and appropriate exposure times are practiced, these
problems will be prevented. So, not only the ultimate temperature reached in the tissue
interaction with the laser energy is of concern, but also the temporal duration of this
temperature increase plays a significant role for the induction of desired tissued effects,
and the inhibition of irreversable tissue damage. For nano- and picosecond pulses (that
today’s dental lasers cannot achieve), heat diffusion during the laser pulse would be
negligible. (4)
The power density of the beam is determined by the peak power generated by
the laser, divided by the area of the focused beam. This means that the smaller the diameter of the fiber used to deliver the energy (200um, 400um, 600um), and the
closer the fiber is to the tissue (i.e., a smaller “spot size”, not touching the tissue), the
greater the power density (amount of emitted photons per square mm of the beam) and
the greater the thermal interaction. With a non-contact “clean” fiber tip, the two most
important considerations are the spot size of the beam, and the distance of the fiber tip to the tissue. If the dental near-infrared lasers are used in the “contact mode” with a “hot-tip” fiber, the energy delivery, and hence the photobiology, will substantially change. These changes need to be understood by the practitioner, and are discussed in the next section . (6,7)
Yet another parameter which Niemz did not examine, but is commonly employed during dental procedures because it is readily available in the operatory, is the effect that a cooling water spray has on the tissue effects and photobiology. A hot tip laser procedure can be modified with successful results and minimal damage to the adjacent healthy tissue by applying the hot tip in contact with a concomitant water spray applied to the surgical site from an air/water syringe. The water cools the tissue to help control the heat buildup in the tip, and also flushes some of the coagulum away from the tip so that it is a modified “hot tip”. Whether the procedure is being performed in a dry field or with a water spray has significant effects on how the procedure is performed, and if the variations are understood, successful results can be accomplished with both techniques. However, this irrigation and cooling cannot be accomplished, if the tip is placed within a periodontal of periimplant pocket for sulcular curettage. Hence, during these “closed procedures” the power must not exceed 1 to 1.4 watts, with the practitioner never staying in one spot for more than a second or two, during treatment. (Figure 4)
Figure 4 Blackbody “Hot Tip” in Periodontal Pocket
Quantum Changes in the Fiber Tip with Contact “Hot Tip” Techniques
In addition to being the means to deliver laser photons to a target tissue, the silica fibers at the tip of the diode laser device can act as a “hot tip” cutting device. When an activated, unclad fiber tip comes in contact with tissue and fluid, debris will immediately accumulate on the tip. This debris will absorb the intense infrared laser energy propagating through the fiber, which will cause the tip to heat and immediately carbonize the detritus. As the energy from the infrared laser photons continue to be absorbed by this newly carbonized tip, the tip will become red hot (above 726◦ C). (8) Once this occurs, the tip of the fiber (becoming a “black body radiator”) will generate a secondary visible optical emission as it becomes incandescent and glows. (Figs 5,6) As more photons from the near infrared dental laser continue to bombard the black, carbonized tip and are absorbed by the organic debris, there is a rapid increase in temperature at the tip. (9,10)
Figure 5
Tissue Deritus on Tip
Figure 6 “Blackbody Radiator”
It is this intense heat of the carbonized and glowing fiber tip that is known as the “hot tip” for diode laser cutting procedures. With this “hot tip” technique the penetrating laser energy is substantially reduced, and the photobiology and laser-tissue interaction is profoundly different from what is found when using a non-carbonized fiber that emits only the primary emission, near-infrared photons. (These realities need to be clearly understood by the practitioner so that safe and predictable surgical procedures can be realized with these lasers. (7)
“Hot Tips” and Black Body Radiators
To understand the thermodynamic and photobiologic ramifications of the intense heat and subsequent carbonization of the fiber tip, a short review of how black solids absorb and then reemit electromagnetic energy is needed. In the mid 1800’s Gustav Kirchoff proposed a rule for radiant energy that stated “a hot opaque substance emits a continuous spectrum of radiation.” He clarified that black solid objects “glow” and emit light when heated. This phenomenon is referred to as “blackbody emission”. (9) In October 1900 Max Plank, by examining the available experimental data concerning the emission of heat and light from high temperature solids, began our modern understanding of Quantum Mechanics. (9,10) Plank’s analysis revealed some fundamental rules of Quantum Mechanics (Black Body energy release arises from thermal radiation and thermal excitation of atoms). Dentists using diode lasers in the contact mode can appreciate a few of the quantum realities about the “hot tips” (blackbodies) that are part of the cutting process. (9,10)1)Theoretically, a blackbody is an object that absorbs all light. (ie the carbonized tip absorbs a large percentage of the infrared photons being emitted from the laser.)
2)As the carbonized tip continues to absorb laser photons, it heats up. (ie. the longer the laser is firing into the “hot tip”, or the higher the output energy, the hotter the tip will be)
3)The energy and peak wavelength of emitted photons depends on the temperature of the tip. (ie. The hotter the tip becomes, the more total light infrared, visible, and ultraviolet will be emitted from the tip.)
4)The heated tip emits light (photons) in a continuous spectrum at infrared, visible, and ultraviolet wavelengths. (ie. No longer just the single infrared wavelength from the primary emission of the laser.)
5) Hotter objects are brighter at all wavelengths. (9)
Figures(7,8)
Although a true blackbody radiator is only theoretical, many materials behave in a similar manner when they are heated by an external energy source. An example of another common “blackbody radiator” is the tungsten filament in a conventional light bulb. The filament glows and becomes incandescent as it is heated by an electrical current, and becomes brighter as it gets hotter. (11,12)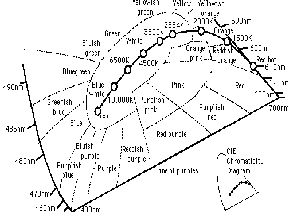
Figure 7 CIE Diagram in Degrees K
Figure 8 “Incandescent Blackbody Radiator”
Tissue Thermodynamics of “Hot Tip” Emissions
To accomplish safe and predictable surgical procedures with a “hot tip” and a near infrared dental diode laser, the clinician must be familiar with the very narrow therapeutic window afforded by the tip’s thermal interactions with tissue. When radiant optical and thermal energy is applied to biological tissues with a “hot tip”, the temperature of the contact area rises immediately. At 45◦ C, the tissue becomes hyperthermic. At 50◦ C., there is reduction in cellular enzyme activity and some cell immobility. At 60◦ C, proteins denature, and there is evidence of coagulation. At 80◦ C, cell membranes are permeable, and at 100◦ C, water and tissue begin to vaporize. (13)
If the temperature increases for 2 to 5 seconds beyond 80◦ C, there will be irreversible damage to the mucosa, bone, periodontal, and dental structures. (4) These considerations are of direct importance for contact tip procedures such as a gingivectomy, gingivoplasty, frenectomy, incision and drainage, removal of a fibroma, and sulcular currettage. (13)
Surgical Objectives with “Hot Tips”
The objective when using a diode laser with a “hot tip” is to generate sufficient thermal energy at the tip to cause immediate tissue vaporization and ablation limited to the line of the incision. To properly accomplish this, the tissue must be rapidly heated to several hundred degrees Celsius at the contact point of the tip. A diode or Nd:YAG laser can readily accomplish this if properly used in a contact mode. As the optical and thermal energy (of the secondary blackbody emission) is directly transferred to the tissue in the vicinity of the tip, a controlled vaporization ensues. ( Figures 9,10)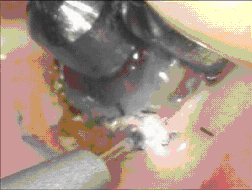
Figure 9 Contact Vaporization Figure 10 Superficial Cautery
During these procedures, it is imperative to keep treatment contact intervals in any one spot relatively short (1 to 2 seconds), since limited exposure to the tip will minimize damage to the peripheral tissues. The heat will be transferred deeper into the tissues via heat conduction, and can be rapidly dissipated by the tissues if there are very short periods of contact. If the contact exposure time is too long (more than 2-3 seconds in one area), the ability of the tissues to dissipate heat is overcome, and irreversible damage occurs to non-target tissues.Photobiology Differences with Contact “Hot Tips”
As stated, in contact mode a large percentage of the near-infrared photons (the primary emission of the laser) are absorbed by the blackbody tip and carbonized coagulum. As a result, the emission, and hence penetration and absorption of these primary (single wavelength) infrared photons generated from the laser, are greatly decreased. Consequently, the ability of the laser to coagulate in the deeper tissues is now greatly attenuated. Therefore, the size of the resulting coagulation zone associated with an incision with the tip is dependent on the exposure time of the “hot tip” to the tissue and the heat conduction from the tip to the tissue. These greatly decreased primary emissions of the laser through a carbonized tip were studied in detail by Grant et al, (8) as they specifically looked at the “fiber interaction” during contact laser surgery. Grant showed that with tissue deposits at the tip of the fiber absorbing larger amounts of laser light, immediate carbonization occurs. The carbonization of the fiber tip leads to an increase in temperature, and this can result in significant damage to the optical quality of the fiber (the temperature spikes to greater than 900◦ C). They also found that once the carbonization of the tip occurs, the tip no longer functions as an adequate light guide. The laser will no longer adequately photocoagulate, but rather it incises and cauterizes the tissue because of the intense heat at the tip. (8) Figures (10,11)
Figure 10 (clean un-carbonized)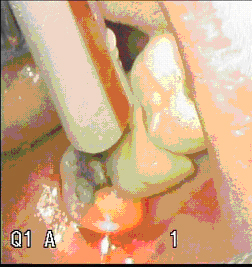
Figure 11 Carbonized Fiber
It is important to remember that the glass portion of an optical transmission fiber consists of two regions – the core that runs through the center of the strand, and the cladding that surrounds the core. The cladding has a different refractive index than the core, and acts as a mirror that causes the laser light to reflect back into the core during its transmission through the fiber. (12) Furthermore, longer lasing times and higher power drastically reduces the forward power transmission of the laser light, as the fiber tip sustains more and more heat induced damage. (8) When testing a 360micron fiber with a 830nm diode laser at 3 watts CW, this author (testing with a laser power meter) found that an immediate 30% loss of forward power transmission is observed with fiber carbonization from tissue detritus. Further loss was observed as lasing time continued and tissue debris accumulated.
This phenomenon was examined in vivo by Willems and Vandertop. (14) Using diode and Nd:YAG lasers, conventional fiber tips and coated fiber tips were compered for ablation efficiency in rabbit cerebral tissue. With the conventional fiber tips, histology and thermal imaging demonstrated deleterious effects deep into the tissue. When using the coated fiber tip, they reported that almost all laser light was transformed into thermal energy (as the tip carbonized), and instantly produced ablative temperatures at the tip itself. Further, they reported that ablation was observed at low energy and power (1 W for 1 second) with thermal effects restricted only to the superficial structures.(14) This restriction of thermal effects to superficial structures can be explained, as the forward power transmission of the laser light is attenuated when a larger percentage of the primary emissions of the laser are absorbed by the tip. As a result, the optical transmission qualities are damaged. (8) Also of significance, as the quality of the fiber transmission diminishes as a result of damage to the tip, the energy, focus, and homogeneity of the energy being transmitted from the tip is affected. The primary energy that is still available for forward power transmission out of the tip is far less efficient for tissue penetration and photocoagulation. These are the important and fundamentally different biological consequences associated with diode lasers in the contact or non-contact modes.Medical/Clinical Applications and Safety of “Hot Tips” with Near-infrared Energy Sources
In 1987, Shapshay stated that the Nd-YAG laser contact probe was capable of precise soft tissue ablation with minimal surrounding tissue damage, and was associated with rapid healing of the tissues (trachea and bronchi). (15) In 1991, Absten described the use of hot contact tips with Nd:Yag lasers in gynecology for endometrial vaporization and ablation.(16) Recently, in a study evaluating the thermal damage to the interior walls of veins with 600um fibers in endovenous laser treatment, no major differences could be detected between the three diode laser wavelengths of 810nm, 940nm, and 980nm. The laser wavelength interaction with the blood was strong enough to transfer the optical energy completely into heat at all wavelengths, even with new, uncarbonized fibers. If carbonization occurred, the tip preferentially absorbed the laser energy, causing extremely high temperature generation and a “hot tip’. These studies strongly suggest that once a fiber tip is carbonized (and becomes a secondary blackbody emitter), subtle wavelength differences are not critical. (17)
Conclusion
The optical fiber tips used with near infrared lasers (800nm-1064nm) experience heat induced carbonization almost immediately upon contact with oral tissues and/or blood. The carbonization is thermally driven, and causes a dramatic degradation of the forward power transmission potential from the tip, as the tip absorbs the primary infrared photons from the laser and becomes red hot and incandescent. Upon carbonization, this tip can be referred to as a blackbody emitter of secondary radiation (ultraviolet, visible, and infrared light), and has a thermal interaction and photobiology distinctly different from what occurs with clean, uncarbonized non-contact fibers. It is no longer a deep photocoagulator.
This hot tip offers the clinician certain advantages over other surgical modalities, as it will adequately cauterize tissue within its immediate periphery, if used correctly. This author would recommend not performing procedures over 3 watts in the CW mode with diode lasers, unless the surgeon is very skilled, and has adequate assistance with cooling irrigation. Also, for closed procedures (in the sulcus) 1-1.5 watts is the maximum energy suggested with rapid movement of the fiber so as not to build excessive heat up in one particular area.Dr. Bornstein received his Bachelor of Science in Biochemistry from the University of Vermont in 1988, graduated from Tufts University School of Dental Medicine in 1992, and the Maimonides Medical Center General Practice Residency program in Brooklyn, NY in 1993. He has been using lasers in his dental practice since 1995, and practices general, implant and laser dentistry in Natick, Massachusetts, while also operating the Metrowest Maxillofacial Imaging Center at the same location.
Disclosure: Dr. Bornstein is the Chief Science Officer for NOMIR Medical Technologies, a company focused on novel laser based solutions for eradicating Biofilms and Bacterial Disease entities. NOMIR currently has multiple patent pending laser based technologies, in first phase testing, that Dr Bornstein plans to bring to the FDA for human efficacy trials in the near future. He has also provided consulting services to OpusDent USA concerning matters of Photobiology and Laser T issue Thermodynamics, and currently owns two combination Er:YAG/CO2 dual wavelength lasers and two 830nm diode lasers, all purchased from Opusdent USA. He also owns one 808nm Diode laser from ZAP Lasers USA. Dr. Bornstein can be reached for consulting or speaking purposes at drericdmd@mindspring.com
REFERENCES
1. Lanigan, S: Lasers in Dermatology, London, Springer, pp 99-103, 2002
2. Venugopalan V, et al: Pulsed Laser Ablation of Tissue: Surface Vaporization or
Thermal Explosion?, Wellman Laboratories of Photomedicine, Harvard Medical
School, SPIE Vol. 2391, 1995.
3. Clayman L and Kuo P, Lasers in Maxillofacial Surgery and Dentistry,
New York, Thieme, pp. 19-28.
4. Niemz, M: Laser-Tissue Interactions. Fundamentals and Applications, Berlin,
Springer, pp45-80, 2002
5. Bornstein E. and Lomke M., Safety and Effectiveness of Dental Er:YAG Lasers: A
Literature Review with Specific Reference to Bone, Dentistry Today, Vol 22 No 10,
pp129-133, October 2003
6. Verdaasdonk, R. and Swol, C., Laser Light Delivery Systems for Medical
Applications, Phys. Med. Biol., 42 (1997) 869-894.
7. Janda, P. et al., Comparison of thermal tissue effects induced by contact application of
fiber guided laser systems, Lasers in Surgery and Medicine, Volume 33, Issue 2,
pp 93-101, 2003.
8. Grant, S. et al, Degradation-Induced Transmission Losses in Silica Optical Fibers,
Lasers in Surgery and Medicine, 21:65-71 (1997)
9. Kuhn, T: Black-Body Theory and the Quantum Discontinuity, 1894-1912, Chicago,
The University of Chicago Press, 1978
10. Planck, M: The Theory of Heat Radiation, New York, Dover Publications Inc. 1991
11. The Chemical Educator, 3 / Vol. 3, No. 4, Appendix, Springer-Verlag, New York,
1998
12. Serway, R., Physics for Scientists and Engineers with Modern Physics, Second
Edition, Philadelphia, Saunders College Publishing, pp 812-814, 1986
13. Rossman J., Lasers in Periodontics, Journal of Periodontology, 2002; 73” 1231-1239
14. Willems PW, Vandertop WP et al, Contact laser-assisted neuroendoscopy can be
performed safely by using pretreated ‘black’ fibre tips: experimental data, Lasers
Surg Med. 2001;28(4):324-9.
15. Shapshay SM, Laser applications in the trachea and bronchi: a comparative study of
the soft tissue effects using contact and noncontact delivery systems, Laryngoscope.
1987 Jul;97(7 Pt 2 Suppl 41):1-26.
16. Absten GT, Physics of light and lasers, Obstet Gynecol Clin North Am. 1991
Sep;18(3):407-27
17. Proebstle, T.M., et al. Thermal Damage of the inner Vein Wall During Endovenous
Laser Treatment: Key Role of Energy Absorption by Intravascular Blood, Dermatol
Surg 2002; 28:596-600.
ASISpectatorHi Ron,
Thanks for the post. Great info.
Andrew
AnonymousGuestToo bad the pictures didn’t come thru a little clearer.
Article was posted w/ permission. Thanks Eric and congrats on the article and the newborn!.
lagunabbSpectatorThanks Ron and EB for the article. It is very nice. Just a couple of questions:
In terms of usage, how much work do you do in the activated mode versus optical mode when using these lasers?
I was confused about why BBR was needed to explain the activated mode. How much of the energy from the hot tip is actually transmitted to tissues by far-infrared radiation compared to heat-conduction? I understand the part about convertion of optical energy to heat and infrared rays. However, BBR can exist even for very cold masses (anything above absolute zero). That’s how astronomers detected the newest planet.
Thanks for any comments.
(Edited by lagunabb at 7:46 am on Mar. 19, 2004)
dkimmelSpectatorRon,
Eric does some great articles. It sure would be great if he did some lecturing. It would be worth a plane ride to hear him.David
SwpmnSpectatorQUOTEIn terms of usage, how much work do you do in the activated mode versus optical mode when using these lasers?Thanks for any comments.
Ray:
Personally I use an 810nm diode laser almost exclusively in an activated tip mode Continuous Wave 0.9 to 1.25 Watts for retraction of gingiva/hemostasis around crown/bridge preparations. I have utilized both 300 and 400 micron diameter fibers.
Al
SwpmnSpectatorComparison/Contrast of Electrosurge/Radiosurge with Activated Fiber Tips of 810-830nm Diode Lasers
From my studies and Dr. Bornstein’s paper it appears that an activated(carbonized) diode laser tip vaporizes gingival tissue via heat with minimal input from the specific 810-830 nm wavelengths. In other words the activated diode laser tip acts as a piece of “hot glass” which melts away tissue.
So, what if any difference exists between an activated diode laser fiber tip and the tip of an electrosurge unit?
What’s the difference between electricity vs. a specific wavelength emitted by a laser? For a clinical example, why can a patient get a “shock” from an electrosurge unit yet I’ve never had this happen from my diode laser? Even on thoroughly anesthetized patients this can occur with electrosurge – mandibular molars were often challenging for me in my pre-diode years.
With the diode laser in an activated mode, we commonly retract the gingiva in areas that are not completely anesthetized with no response from the patient. For example, palatal of maxillary crown preps and lingual of mandibular premolars anesthetized via the mental foramen. Perhaps this could be accomplished with electrosurge but guess I was always too scared to try it on a patient.
Now for the big question:
Has anyone else experienced or can anyone explain to me why it seems that I get far greater control of hemorrhage around crown/bridge impressions with an activated diode laser tip(one year) when compared to over ten years usage of electrosurge on coagulation settings? Aren’t they both just two hot wires?
Al
SwpmnSpectatorFor those still interested in this old thread which I either overlooked or lost due to server issues, I was able to obtain some clarification and answers to my questions:
1) The objective when using an activated diode laser tip on dental soft tissues is not my oversimplified term “melting” but immediate tissue vaporization limited to the line of incision. Water and tissue vaporization begin at 100C while an activated diode laser tip operates at several hundred degrees Celsius(perhaps 700-900C).
2)Electricity in electrosurge and the specific 810-830nm wavelength emitted by a diode laser are two completely different forms of electromagnetic energy. Electrons from electrosurge are conducted by water, so the water in human tissue propagates electricity and can give the patient a “shock”. That’s why we use the grounding plate under the patient’s back. The infrared photons emitted by the diode laser are absorbed by hemoglobin in tissue and thus do not travel throughout the patient’s body.
3) With an activated/carbonized diode laser tip, there is a drastically reduced forward power transmission of the specific 810-830nm wavelength. Much of the laser energy is absorbed by the carbonized tip and converted to heat. However, there is still some forward progression of primary photons(810-830nm) coming out of the fiber tip. One obtains a deeper coagulation with the diode tip as there are still some photons absorbed by blood products deeper in the tissue than would account for simple heat conduction from a “hot tip”. This would explain my clinical finding of superior hemorrhage control using a diode laser around fixed prosthetic preparations when compared to electrosurge.
Al
SwpmnSpectatorReference:
The clarification posted in the immediately previous thread is credited to the author of the paper, Eric Bornstein DMD.
Al
-
AuthorPosts
